Thing need consider when find device history record fda?
When you looking for device history record fda, you must consider not only the quality but also price and customer reviews. But among hundreds of product with different price range, choosing suitable device history record fda is not an easy task. In this post, we show you how to find the right device history record fda along with our top-rated reviews. Please check out our suggestions to find the best device history record fda for you.
Best device history record fda
1. Agent GXP FDA Part 11 Guidebook: The FDA Regulations on Part 11, Electronic Records and Electronic Signatures, For Pharmaceutical, Medical Device, ... and GCP (Good Clinical Practices) (Pt. 11)
Description
Agent GXP FDA Part 11 teaches the FDA regulations on electronic signatures and records in the context of a spoof on a hostage rescue supervised by Pharm Mission Control. It is taught mostly through interactive questions and interviews with Agent GXP. The many difficult regulations of Part 11 are broken down into episodes that make the learning more memorable. This thorough course will teach you the history of Part 11, the regulations of Part 11, the implementation of Part 11, the applications of Part 11, the ideas behind Part 11 in order to apply them to new situations, and how to prepare for enforcement of Part 11. This is particularly important for both pharmaceutical/medical device manufacturing and clinical research personnel in FDA-regulated industries, and provides an excellent glimpse of the issues that are likely to face HIPAA implementation of electronic records security measures. This course has been used by thousands of people in the pharmaceutical industry. Readers will enjoy the approachable, compact, conversational style of the title. Those readers who wish to have an accompanying program with video and interactivity should also purchase the CD version. This is part of the highly acclaimed UniversityOfHealthCare series on FDA regulations.2. Violations Of 21 CFR Part 820 - Quality System Regulation, Subpart M Records: Warning Letters Issued by U.S. Food and Drug Administration (FDA Warning Letters Analysis) (Volume 15)
Description
Inspection teams often know that a deficiency at one company will often be found at others, so they tend to look for what teams have found in previous inspections. A numerical analysis of past inspections results in the discovery of inspection trends for future inspections. This analysis contains a collection of violations listed in the Warning Letters (WLs) issued by U.S. Food and Drug Administration (FDA) that are available to the public on FDA website, www.fda.gov, as of May 23, 2015. Specifically, the violations included in this analysis are extracted from Warning Letters issued since January 2005. The violations collected here are specifically for failures to meet the requirements described in U.S. Code of Federal Regulations (CFR) Title 21 Food and Drugs, Part 820 Quality System Regulation Subpart M Records. As of May 23, 2015, there were 826 warning letters issued describing violations of Code of Federal Regulations Title 21 - Food and Drugs, Part 820 Quality System Regulation Subpart M Records. Within these warning letters, 1451 violations are listed in this book. The analysis also includes summary of FDA Inspectional Observation issued on Form 483.3. LotFancy Blood Pressure Monitor, Upper Arm Large Cuff (11.8-16.5"), 4-User Mode Electronic Sphygmomanometer (FDA Approved)
Feature
LotFancy blood pressure monitors are the most cost-effective and the most user-friendly brand for clinically-accurate home blood pressure monitoringLarge storage support up to 4 users to store and review last 30 readings each (total 120 reading memory with date and time stamps) - Perfect for a family of 4 with history of high blood pressure and heart disease
Automatically displays the average of your last 3 readings recorded on large LCD screen
Irregular heartbeat detector notifies you if an irregular heartbeat is detected while monitoring
Package includes: 1 blood pressure monitor, 1 large D-Ring cuff (fits ahttps://catalog.amazon.com/abis/product/DisplayEditProduct?marketplaceID=ATVPDKIKX0DER&ref=xx_myiedit_cont_myifba&sku=11C-1851-E.1&asin=B018DLPGII&productType=HEALTH_PERSONAL_CARE#rms 11.8 to 16.5 inches in circumference), 1 power adapter, 1 instruction manual, and 1 carrying pouch
Description
Note: 4 AA batteries not included, but power adapter is included.Steps for an accurate reading:
The fact that blood pressure measurements vary is no surprise. Blood pressure can vary throughout the day and responds dynamically to movements, your diet, and changes in mood.
We suggest taking measurements close to the same time each day. Place the cuff on your exposed arm approximately 1 finger-width (2cm) above the elbow. Make sure the tubing is placed at the center of your arm facing the front, and that the sensor is correctly placed. Pull at the end of the cuff so it is wrapped evenly and firmly around your arm.
IMPORTANT: Please READ the user manual in as much detail as possible regularly to ensure proper use.
Masked Hypertension & White-coat Hypertension:
Your blood pressure can vary depending on changes in posture, exercise, diet, medications, emotions, or sleep. That's why a single blood pressure measurement taken at the doctor's office doesn't always provide accurate information about your health. To get a more accurate assessment of blood pressure, people should perform multiple recordings and record their average.
Specifications:
Accuracy: 3 mmHg (0.4 KPa)
Test Range: 20 mmHg - 300 mmHg (2.7 KPa - 40 KPa)
Pulse Accuracy: 5
Pulse Test Range: 30-180 Beats/Minute
Battery Life: Approximately two months at 3 min/day
Dimensions: 6.7 x 4.5 x 2.5 inches (L x W x H)
Weight: Approximately 1 pound, excluding batteries
Package includes:
1 x Blood pressure monitor
1 x Large Cuff: 11.8-16.5 inches
1 x Power Adapter
1 x User manual
1 x Carry pouch
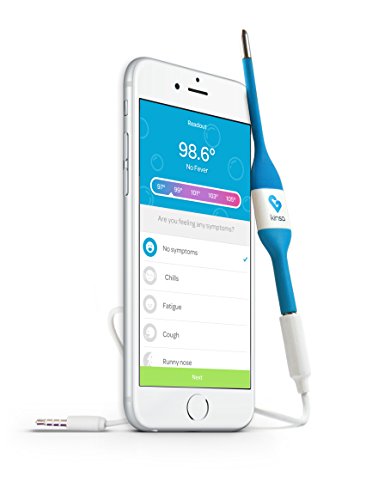
4. Kinsa Smart Stick Digital Thermometer + Storage Pouch, Medical Thermometer with Real-Time Guidance for Baby, Children and Adults-Old Model
Feature
OLD MODEL: Not compatible with all smartphones. Check compatibility at KinsaHealth.com/phones.Get personalized guidance on how & when to soothe symptoms, take meds and call the doctor.
Keep each family member's health details in your pocket for you or your pediatrician. Keeps kids entertained with bubble popping game.
Compatible with all iPhones 5 and up (including iPhone 8 and X -- lightning port adapter needed for iPhone 7 and up) and most Android devices; Kinsa plugs into your iOS or Android device, giving an accurate reading on the free app in seconds.
Pediatrician-recommended fast, accurate, reliable thermometer for all ages with no batteries so it always works.
Our termometro works as a baby thermometer rectally, or with oral and underarm use for people of all ages.
Description
Kinsa's pediatrician-recommended fast, accurate & reliable Smart Stick thermometer, now with storage pouch. Compatible with all iPhones 5 and up (including iPhone X), though please note you will need the lightning port adapter if you have an iPhone 7, 7 plus, 8, 8 plus, or X. Also compatible with most major Android devices. The free app records fevers, symptoms, medication dosages, and other important health data in a time-stamped log. The thermometer takes accurate oral, and underarm temperature readings - as well as rectal readings as a baby thermometer - and its battery-free design means it never dies when you need it most.5. Violations Of 21 CFR Part 820 Quality System Regulation, Subparts H-I: Acceptance Activities and Nonconforming Product: Warning Letters Issued by U.S. ... (FDA Warning Letters Analysis) (Volume 12)
Description
Inspection teams often know that a deficiency at one company will often be found at others, so they tend to look for what teams have found in previous inspections. A numerical analysis of past inspections results in the discovery of inspection trends for future inspections. This analysis contains a collection of violations listed in the Warning Letters (WLs) issued by U.S. Food and Drug Administration (FDA) that are available to the public on FDA website, www.fda.gov, as of May 23, 2015. Specifically, the violations included in this analysis are extracted from Warning Letters issued since January 2005. The violations collected here are specifically for failures to meet the requirements described in U.S. Code of Federal Regulations (CFR) Title 21 Food and Drugs, Part 820 Quality System Regulation Subparts H-I, Acceptance Activities and Nonconforming Product. As of May 23, 2015, there were 609 warning letters issued describing violations of Code of Federal Regulations Title 21 - Food and Drugs, Part 820 Quality System Regulation Subparts H-I, Acceptance Activities and Nonconforming Product. Within these warning letters, 891 violations are listed in this book. The analysis also includes summary of FDA Inspectional Observation issued on Form 483.6. iHelmet Hair Growth Helmet Low Level Light Therapy (LLLT) Hair Loss Treatment for Men & Women FDA Cleared, Painless, Lightweight Hair Regrowth Device with 200 Laser Diodes in 7 Zones + Smart App
Feature
FDA-CLEARED CAP - Thinning, balding, alopecia, and chemotherapy can all result in hair loss for both men and women. You're not alone, and there is a SAFE solution: iHelmet's LLLT hair growth cap. Our FDA-approved cap uses 200 laser diodes to stimulate the scalp and regenerate and regrow hair follicles safely.ZERO SIDE EFFECTS - Imagine laser therapy for hair loss that's totally pain-free and has no side effects. iHelmet's laser hair regrowth hat has all of that and more. Your helmet is comfortably lightweight at around 1 pound, and it can even adjust to your head size.
CUSTOMIZED, TRACKABLE TREATMENTS - Get the treatment tailored to your unique needs! iHelmet features innovative laser technology that creates a personalized protocol for more effective treatments. Your hair growth hat also comes with a Bluetooth-compatible smart app for iOS and Android devices, so you can record your treatment history and track your progress.
AT-HOME HAIR GROWTH - Effortlessly grow strong, healthy hair in the comfort of home with iHelmet. It's a far less expensive and time-consuming alternative to invasive, often painful procedures, and it's totally drug-free! Try laser light for a simple, painless option.
100% MONEY-BACK GUARANTEE - Investing in yourself is the best decision you can make. That's why we promise to refund you in full if you don't begin to see satisfactory results within 6 months of buying your hair regrowth helmet.
Description
We are very excited to offer the new FDA cleared iHelmet LTD200S. The value of this device is the quality it is made with and the technology that goes into it. ThisiHelmet Model 200S, is an effective treatment for men and women suffering from mild to moderate hair loss.
This3rd generation laser hair growth helmet has 200 individually placed lasers throughout the helmet. You will be able to customize treatments, tailoring to your specific hair growth needs with 7 separate treatment zones. The custom zones allowyou to identify your specific troubleareas - your hair thinning pattern - and focus the treatment only where it is needed. This provides a better outcome. Even the movable soft rubber fitting tabs, designed to ensure the helmet fits well on your head for customized comfort, show the level of detail and thought that went into making the iHelmet.
Beyond the iHelmetbuild and design, there has been a great deal of expertise invested into the development of the smartphone app that you use to tailor your treatments and monitor your progress. Through the app you will take baseline pictures, set up your treatment routine and customized zone approach. You will get reminders and guidance on treatment times to ensure you stay on track and you will be able to see the progress by comparing the pictures as you go. John, when comparing this device to others, people are impressed with the quality of build, the professionalism of the app, and the results that they get - and they know the results they are getting is real by the comparisons that are tracked in-app for you.
The product will automatically pause treatment if the sensor detects that the head is not in close proximity to the sensor, and will resume again once close enough. At the end of the treatment, an audible tone beeps to indicate the treatment is over and then the helmet will automatically shut off.
7. Automatic Wrist Blood Pressure Monitor FDA Approved with Portable Case, Two User Modes,180 Memory Capacity Easy to Operate & Store (black)
Feature
Simultaneous readout of systolic/diastolic pressure ,pulse rate and irregular heartbeat detection .WHO Indicator -Blood pressure level color indicator according to WHO classification.
Automatically activates when your wrist is at heart level and indicator lights make it easier to find the correct position.
LCD display : Easy to read,LCD display shows all measurements, date, and time on the same screen.
Double Users:Memory function,Double mode,each of the 90 set of measurements of memory.Easy to know the history blood pressure data.
Description
Specifications:Range: BP: 0~299mmHg; Pulse: 40~199 beats/min
Memory: 2*90 group memory
Accuracy: Pressure: 3mmHg more or less
Average Function: Last 3 times of measurement
Battery: 2*AAA batteries are required (not in package)
LCD Size: 1.4*1.8 inch
Other Functions: Irregular Heart Beat Detection/ WHO Classification Indicator;
Low Battery Indicator/ Time & Date display/ Auto power off
Good to Know:
1.Please keep quiet for 5 -10 minutes and avoid eating, drinking alcohol, smoking, exercising and bathing before taking measurement.
2.Remove watch or other ornaments from the measured wrist.
3.Always measure on the same wrist (normally left wrist).
4.Take measurement regularly at the same time of every day, as blood pressure changes even during the day.
Package and Warranty:
1* Wrist blood pressure monitor;
1* Plastic box.
Warranty: Two years from the date listed on the purchased record
Common factors of wrong measurement:
1. All efforts by the patient to support their arm can increase blood presure.
2. Make sure you are in a comfortable, relax position and do not activate any of the muscles in the measurement arm during measurement. Use a cushion for support if necessary.
3. If the arm artery lies lower or higher than the heart, a false reading will be obtained.
Note
1. Only use clinically approved cuffs!
2. A loose cuff or a exposed bladder causes false reading.
3. With repeated measurements blood accumulates in the arm which can lead to false reading. Consecutive blood pressure measurements should be repeated after 30 minutes pause or after the arm has been held up in order to allow the accumulated blood to flow away.









Recent Comments